Animal Studies
SMS cells were tested on different animal models pertinent to degenerative diseases such as:

Proof Of Concept Studies
SMS cells were tested on different animal models pertinent to degenerative diseases such as:
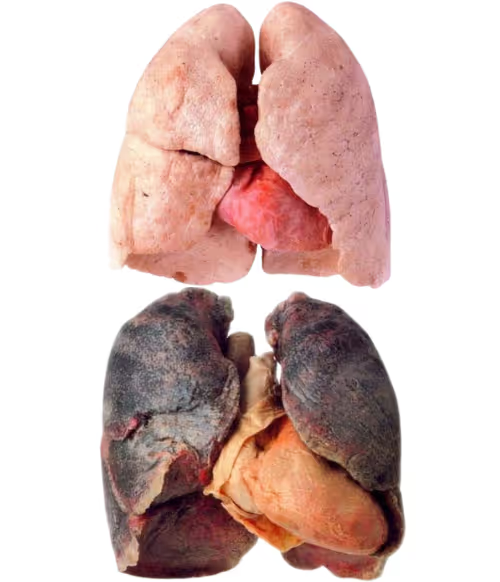
Progressive Lung
Degeneration
COPD
A chronic, inflammatory lung disease that causes obstructed airflow from the lungs. Emphysema (caused by the destruction of air sacs, and alveoli) and Chronic Bronchitis are the most common conditions that make up COPD.
Symptoms include breathing difficulties, cough, mucus, and wheezing. It is caused typically by long-term exposure to breathable irritants. The disease is progressive and currently irreversible. Patients are at increased risk of developing heart disease, lung cancer, and a variety of other conditions.
The model organism used is an elastase enzyme-treated rat. The enzyme damages a significant number of lung air sacs (alveoli) mimicking the case of lung emphysema in patients.
Testing the effect of SMS cells instilled into the lung of this rat resulted in a high percentage of alveoli regeneration using a mere single dose of SMS cells. Different regimens are currently being studied to optimize the healing effect of these cells.
Orthopedic Clinical Animal Studies
SMS cells have been evaluated across multiple orthopedic indications in domestic animals. Early studies already show significant improvement in dogs and horses following either localized or intravenous administration.
The therapeutic effects observed in multiple species using human-derived SMS cells suggest the activation of a fundamental regenerative mechanism driven by small mobile stem cells. Moreover, the ability of SMS cells to treat local pathologies after systemic intravenous delivery implies the existence of a homing mechanism, allowing circulating SMS cells to target and act upon relevant tissues—including those that may be difficult to access or not yet identified as damaged.

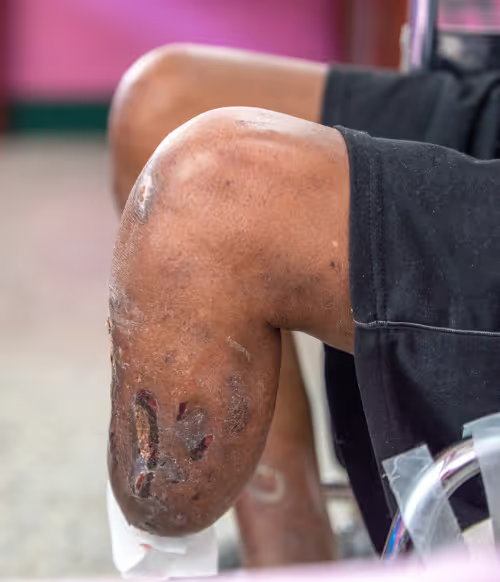
Non-healing Wounds
Diabetic ulcers
Patients with diabetes have a 25% likelihood to develop a foot ulcer. Some of these do not heal using the standard care of medicine, resulting in open wounds that remain as such for very long periods.
These wounds are susceptible to microorganismal invasion that could lead, consequently, to amputation of the limb. Patients with prior amputation have a very low life expectancy.
The model organism used is a mouse strain with a strong diabetic phenotype (BKS.Cg- Dock7m +/+ Leprdb//J) that causes significant delays in skin wound healing. Two excision wounds were inflicted; one was treated, and the other was used as a control. The wounds were splinted to prevent healing by skin contraction.
Applying extracellular matrix (proteins) derived from SMS cells on wounds resulted in a large increase (multiple ratios) in granulation, epithelialization, and capillarization of the healing tissue extracted after 14 days.
Animal Safety Studies
Small Mobile Stem (SMS) cells were tested in different animals and different genders regarding safety. Using large numbers of human SMS cells injected into different regions (organs, tissues) of the animal and by applying various regimens, no adverse effects at tissue, organ, or organismal level were detected.

Stained SMS Cells In Vivo
Visible in red are near-infrared tagged SMS cells that were injected into nude mice. The subcutaneous injection was administered on the right dorsal side of the mouse, above the right hind leg. Whole-body fluorescence and white light images were taken 7 days post-injection. This image shows an overlay of the fluorescent image merged with the white light image.
SMS Cells
Labeled using a near-infrared fluorescent label and subsequently injected into the lung of a mouse. The signal was monitored using the VISQUE™ InVivo Smart-LF instrument, in vivo detection system

References
SMS cells were tested in different animals and different genders regarding safety. Using large numbers of human SMS cells injected into different regions (organs, tissues) of the animal and by applying various regimens, no adverse effects at tissue, organ, or organismal level were detected.
COPD References
- Tomislav M. Jelic (February 12th 2019). Emphysema, Update in Respiratory Diseases, Jose Carlos Herrera Garcia, IntechOpen, DOI: 10.5772/intechopen.83273.
- Ghorani V, Boskabady MH, Khazdair MR, Kianmeher M. Experimental animal models forCOPD: a methodological review. Tob Induc Dis. 2017;15:25. Published 2017 May 2.doi:10.1186/s12971-017-0130-2
- Tanner L, Single A, B: Animal Models Reflecting Chronic Obstructive Pulmonary Disease and Related Respiratory Disorders: Translating Pre-Clinical Data into Clinical Relevance. J Innate Immun 2020;12:203-225. doi: 10.1159/000502489
- Sun Z, Li F, Zhou X, Chung KF, Wang W, Wang J. Stem cell therapies for chronic obstructive pulmonary disease: current status of pre-clinical studies and clinical trials. J Thorac Dis 2018;10(2):1084-1098. doi: 10.21037/jtd.2018.01.46
Wound References
- Tomislav M. Jelic (February 12th 2019). Emphysema, Update in Respiratory Diseases, Jose Carlos Herrera Garcia, IntechOpen, DOI: 10.5772/intechopen.83273.
- Tomislav M. Jelic (February 12th 2019). Emphysema, Update in Respiratory Diseases, Jose Carlos Herrera Garcia, IntechOpen, DOI: 10.5772/intechopen.83273.
- Tomislav M. Jelic (February 12th 2019). Emphysema, Update in Respiratory Diseases, Jose Carlos Herrera Garcia, IntechOpen, DOI: 10.5772/intechopen.83273.
- Tomislav M. Jelic (February 12th 2019). Emphysema, Update in Respiratory Diseases, Jose Carlos Herrera Garcia, IntechOpen, DOI: 10.5772/intechopen.83273.

