Investors
SMSbiotech has discovered and named a new type of stem cell called the Small Mobile Stem cell, which is the core of its patented and proprietary technology.
SMSbiotech Brief Introduction
SMSbiotech, a C-Corp founded in 2015, is a San Diego-based, clinical-stage, biotech company utilizing a novel human stem cell to advance the field of regenerative medicine. SMSbiotech has developed a new, powerful, worldwide, patented proprietary technology based on its discovery of Small Mobile Stem (SMS) cells. Our discovery of SMS cells offers a fundamental solution that addresses the lack of ability of many patients to restore cells and tissues damaged by disease, trauma, and/or aging. As such, it offers a new platform technology that provides practical and efficient solutions in the field of regenerative medicine to unmet needs, targeting cures rather than symptoms of the disease.
Introduction to 0ur Technology
SMSbiotech provides a regenerative solution to lung tissue degeneration. Small Mobile Stem (SMS) cells, which can be bio-manufactured on a large scale, provide an off-the-shelf allogeneic cell therapy platform solution. These cells were originally obtained from healthy human adult blood. They are not genetically modified and are grown while maintaining potency.
Small Mobile Stem (SMS) cells have been shown, in the lab and in animal models, to stimulate lung cells and restore lung air sacs that are damaged. The mechanisms of SMS cells have been studied to a great extent and are unique to these cells. Furthermore, SMS cells are the only cells that can be administered non-invasively and directly to the lung—by inhalation using a nebulizer. Additionally, Small Mobile Stem (SMS) cells interact with other cells of different tissues and stimulate the repair and regeneration of various organs, proving their value as a platform technology.The strong regenerative effects of human-derived SMS cells can be observed across multiple species, suggesting an underlying universal principle of tissue regeneration.
What sets SMSbiotech apart from other biotech companies?
The discovery, development, patenting, and commercialization of Small Mobile Stem (SMS) cells set SMSbiotech apart from other biotech companies. SMS cells possess unique characteristics that provide clear advantages over other stem cell therapies. Unlike mesenchymal stem cells (MSCs), SMS cells are naturally small, highly mobile, and sourced from peripheral blood rather than bone marrow or solid tissue. This allows for scalable production from a single donor, with little to no limitations on dose size.
They are stable for more than 27 passages in culture and can remain viable in suspension at standard refrigeration temperatures (2–8°C) for up to 26 days—eliminating the need for freezing and thawing. SMS cells can be administered not only through injection but also via inhalation, making them suitable for respiratory indications such as COPD. They demonstrate strong and selective binding to target cells, stimulate repair mechanisms in other stem cells, and act through multiple mechanisms, including direct binding and secretion-driven gene expression changes.
In addition, Small Mobile Stem (SMS) cells avoid key drawbacks of MSCs: they do not clog capillaries, produce little to no inflammation, and retain effectiveness for prolonged periods in vivo. Collectively, these features make SMS therapy more effective, safer, and far more practical to deliver and scale globally. Furthermore, SMSbiotech’s platform provides early access to revenues worldwide through compassionate use (Early Access Programs), creating both a clinical and commercial advantage.
What is the Main Targeted Disease?
Chronic Obstructive Pulmonary Disease (COPD), which encompasses emphysema and chronic bronchitis, is a deadly progressive lung disease for which there is currently no cure. Patient’s lungs gradually deteriorate, as does their quality of life until death. It is the 3rd leading cause of death. More than 16 million U.S. citizens have COPD (400 million worldwide), affecting at least one of every four families. Given that current treatments only address symptoms, experts believe a mere 10% improvement in lung function would be a great achievement in medicine.Chronic Obstructive Pulmonary Disease (COPD), which encompasses emphysema and chronic bronchitis, is a deadly progressive lung disease for which there is currently no cure. Patient’s lungs gradually deteriorate, as does their quality of life until death. It is the 3rd leading cause of death. More than 16 million U.S. citizens have COPD (400 million worldwide), affecting at least one of every four families. Given that current treatments only address symptoms, experts believe a mere 10% improvement in lung functions would be a great achievement in medicine.
What are the Future Verticals?
SMSbiotech's proprietary stem cell technology is a platform that targets multiple other chronic diseases, as shown in animal studies.

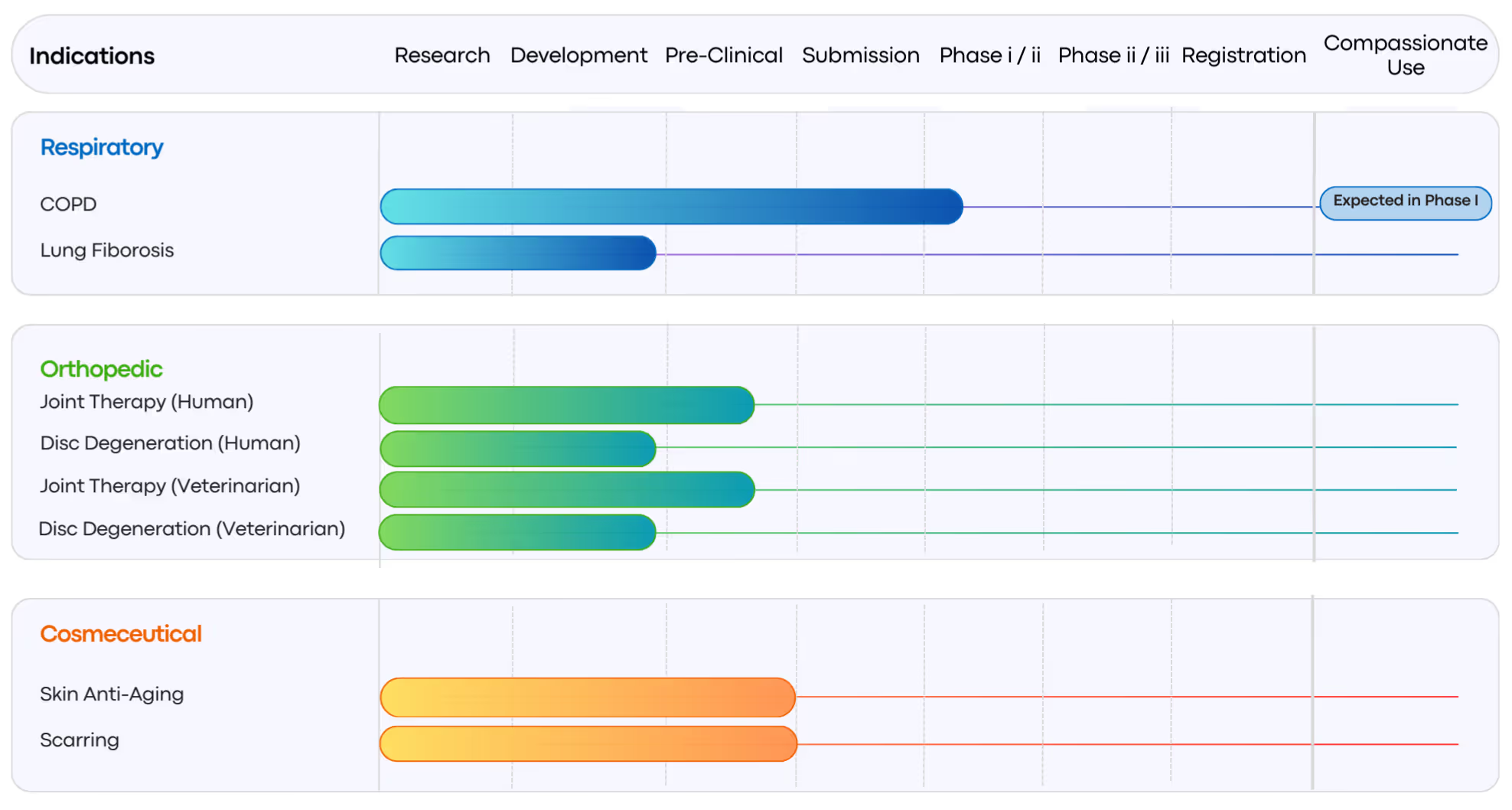
What is the Therapeutic Pipeline?
SMSbiotech’s therapeutic pipeline spans multiple indications in respiratory, orthopedic, veterinary, and cosmeceutical applications. In respiratory care, programs targeting COPD and lung fibrosis are already in Phase I clinical trials. Current programs are at various stages, ranging from research and pre-clinical development to anticipated clinical submissions, with compassionate use pathways under consideration.
In addition to respiratory diseases such as COPD, Small Mobile Stem (SMS) cell therapy is also being developed for widespread chronic conditions, including orthopedic indications in both humans and animals (dogs and horses). Beyond these, SMSbiotech’s scientists and global research collaborators are actively exploring additional therapeutic applications, further expanding the platform’s potential.
What is Your Pathway to Revenue?
The earliest revenues are expected to be generated through compassionate use treatments, which could begin as early as the Phase I COPD clinical trial. This milestone not only represents the first opportunity to bring SMS cell therapy to patients in need but also marks the beginning of SMSbiotech’s revenue-generating activities.
To support this growth and ensure scalability, SMSbiotech is expanding global access to Small Mobile Stem (SMS) cell therapy by enhancing its in-house biomanufacturing capabilities. This investment in production capacity positions the company to meet increasing demand as clinical programs advance and new therapeutic applications emerge.
SMSbiotech is providing worldwide increasing access to Small Mobile Stem (SMS) cell therapy by enhancing its own capacity for cell biomanufacturing.

What is Your Regulatory Path?
SMSbiotech has achieved a significant milestone by receiving approval from the Australian TGA, the equivalent of the US FDA, to proceed with a Phase I clinical trial for its innovative COPD treatment. We launched our Phase I COPD trial in April 2025 under Australian TGA and HREC approvals, and in July, successfully dosed our first two sentinel patients without any adverse events observed. Following FDA Pre IND recommendations, the trial will enroll 18 COPD patients, and early safety data will open the door to Compassionate Use (Expanded Access) programs for severe COPD patients globally. This initiative not only accelerates clinical validation but also enables early revenue generation. SMSbiotech will pursue compassionate use authorization both internationally and in the United States. This will be followed by an application in the U.S. to initiate a Phase 2 clinical trial of its cell therapy for COPD.
Adoption of COPD Therapy:
SMS cells are likely to be easily adopted by the medical community and the patients:
- Small Mobile Stem (SMS) cells are adult stem cells; these cells are largely thought to be safe, contrary to embryonic or iPSCs. This will reassure patients, physicians, and regulators.
- The clear mechanism of action that was demonstrated in vitro will help create an understanding and acceptance of these cells by the scientific and medical community.
- The therapy intends to address the actual disease, that is, the damaged lung tissue, not merely the symptoms of the damage.
- The therapy can be reasonably priced, given the mentioned advantages related to the central mode of production.
- The cells will be applied non-invasively through inhalation to the lung directly, that is, the location of the damaged tissue.
- The Small Mobile Stem (SMS) cells are not genetically manipulated; such cells are potentially unsafe and may harbor dangerous mutations. SMS cells do not require genetic manipulations; their actions follow a naturally optimized process of stimulating regeneration.
- The therapy addresses an indication that is a deadly disease, causing a long-term worsening of suffering that could extend for decades. This suffering includes not only patients but also family members and loved ones who are related to the patient.
- The effects of the Small Mobile Stem (SMS) cell therapy should be observable by analyzing lung function and detecting the lung structural changes, a clear, non-subjective manifestation of the efficacy of the drug.
- Small Mobile Stem (SMS) cell therapy is being explored for treatments in multiple diverse applications with initial significant success.
What Are Current Relevant Markets?
Chronic Obstructive Pulmonary Disease (COPD)
In 2020 COPD caused a $50 Billion economic burden in the US. The global COPD treatment market was valued at US $19.8 Bn in 2021. The global COPD therapeutic market is expected to grow at a 5% CAGR from 2019 to 2027. Considering the size of the market, acquiring a 2.5% US market share would generate $500 million in sales and $ 350 million in EBITDA for SMSbiotech. The company would then be worth $ 3.5 billion. Current treatments target COPD’s detrimental symptoms but not the fundamental cause of the disease. They may slow progress but do not reverse or cure the disease. Current treatments include short-acting and long-acting Bronchodilator Inhalers (albuterol, levalbuterol, and ipratropium) and Corticosteroids. Non-drug treatments include supplemental oxygen and surgery such as lung volume reduction and lung transplantation. Examples of companies that provide bronchodilator inhalers: GlaxoSmithKline, Merck, AstraZeneca, Boehringer- Ingelheim.
Degenerative Knee Disease (DKD / Osteoarthritis of the Knee):
Knee osteoarthritis is among the most common degenerative joint diseases, impacting more than 250 million people worldwide. The global knee osteoarthritis treatment market exceeded USD $5 billion in 2021 and is expected to reach over USD $9 billion by 2030, at a CAGR of ~7%. Current treatments include nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroid or hyaluronic acid injections, and knee replacement surgery, which remains the standard of care for advanced disease. Companies active in this sector include Zimmer Biomet, Stryker, Smith & Nephew, and Johnson & Johnson.
Degenerative Disc Disease (DDD):
Degenerative disc disease is a leading cause of chronic back pain, affecting millions worldwide and representing one of the largest segments of the global orthopedic and spine market. The global spinal degenerative disease treatment market was valued at over USD $12 billion in 2022 and is projected to grow at a CAGR of 6–7% over the next decade, driven by aging populations and demand for regenerative therapies. Current treatments—such as pain management drugs, steroid injections, and spinal fusion or disc replacement surgery—focus on symptom relief rather than regeneration. Major players in this market include Medtronic, Stryker, and Johnson & Johnson’s DePuy Synthes.
What is the Goal of SMSbiotech as an Innovative Company?
SMSbiotech technology will lead to the development of more efficient, disease-changing, and relevant medicines, serving a broad spectrum of patients and reducing the burden on healthcare. This will create a platform technology that will allow for multiple exists to investors through licensing, royalty deals, and partnerships with major players in the industry.
Who are your Board Members?





Who can currently invest in SMSbiotech?
SMSbiotech is a privately held company, and only accredited investors can invest. One of the following items must apply:
- Is a natural person and has a net worth, either alone or with the Purchaser’s spouse, of more than $1,000,000, excluding the value of the primary residence of such natural person and excluding the amount of debt secured by such primary residence, up to the estimated fair market value of the property.
- Is a natural person and had income in excess of $200,000 ($300,000 including income of spouse) during each of the previous two years and expects to have income in excess of such amounts during the current year.
- The entity/the Investor is a corporation, LLC, partnership, business trust, or tax-exempt 501(c)(3) organization that has greater than $5,000,000 in total assets and was not formed for the specific purpose of investing in SMSbiotech, Inc.
- The entity/the Investor is any trust that has greater than $5,000,000 in total assets, and is directed by a person with such knowledge and experience in financial and business matters that such person is capable of evaluating the risks and merits of an investment in SMSbiotech, Inc., and that was not formed for the specific purpose of investing in SMSbiotech, Inc.
- The entity/the Investor is a private business development company as defined in Section 202(a)(22) of the Investment Advisors Act.
- The entity’s/the Investor’s equity owners are all accredited investors. This category may apply even if the entity was formed for the specific purpose of investing in SMSbiotech, Inc.
- The entity/the Investor is a bank, savings and loan association, registered broker or dealer, insurance company, or registered investment company.
Learn more about this opportunity, click to invest!
How does the company plan to use the proceeds from investments?
- Phase I trial in Australia
- Bio-manufacturing site scale-up of cGMP-compliant SMS cell production
- Regulatory consulting and filing
- Pursuing additional human indications
- Worldwide, revenue-generating compassionate use applications of the platform technology
- Pursuing animal health indications, especially those relevant to human diseases
- U.S. military-led animal study collaboration
- Expansion and protection of intellectual property
- Operating and expansion costs
How does the company plan to generate revenue before receiving full commercial approval?
SMSbiotech plans to apply for compassionate use worldwide. This will allow the company to generate early revenues prior to commercial approval. SMSbiotech has obtained approval from a U.S IRB for Expanded access (compassionate use) applications in COPD in the United States. Small Mobile Stem (SMS) cell therapy addresses unmet needs in medicine and is therefore qualified for the application of compassionate use.
SMSbiotech will seek early access for treatment of patients in several countries with different regulations that govern stem cell therapies.
What are the company's plans for biomanufacturing?
SMSbiotech is establishing its own cGMP (current Good Manufacturing Practice) biomanufacturing facility in San Diego County. Preparing the facility started development in June 2025 and is planned to be fully established in Q1 2026. Additional biomanufacturing facilities will be built by SMSbiotech outside the United States.
How is SMSbiotech's intellectual property (IP) protected?
SMSbiotech, in collaboration with Knobbe Martens—one of the nation’s leading intellectual property law firms—has filed fifteen patents worldwide, with seven already granted as of September 2025. These patents cover the composition of SMS cells, methods of use, broad applications, and manufacturing processes. All intellectual property was discovered and developed entirely in-house, ensuring full ownership and control.
Protecting our technology is a top priority. Our IP strategy is multi-faceted and designed to maintain a strong competitive advantage:
- Patent Portfolio: Filed and managed in partnership with Knobbe Martens, covering SMS cell composition, applications, and manufacturing methods.
- Trade Secrets: Years of proprietary research and development have established extensive trade secrets, including specialized know-how in isolation, biomanufacturing, and quality control. These safeguards create a significant barrier to entry for competitors.
How did the stock price of SMSbiotech historically perform?
The following graph illustrates the stock price performance over 10 years of the company's growth
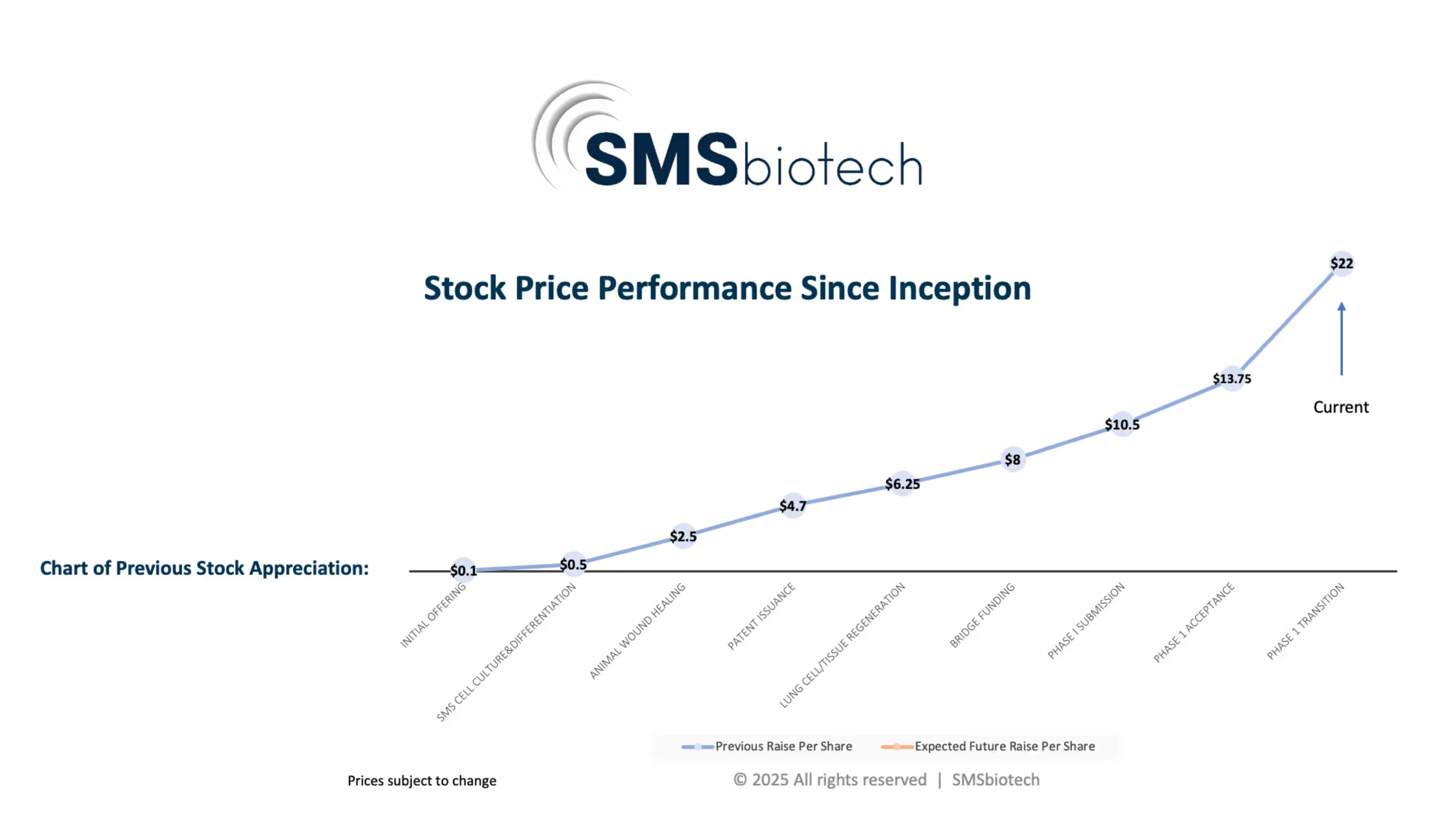
Find below distinguished milestones:
Initial Offering
SMS Cell Culture & Differentiation
Animal Wound Healing
Patent Issuance
Lung Cell/Tissue Regeneration
Bridge Funding
Phase I Submission
Phase 1 Acceptance
Phase 1 Transition
400-to-1 Forward Share Split
Bio-manufacturing Site Complete
Human Safety Data
Human Efficacy Data
Compassionate Use
Partnering/Licensing
BLA/FDA Approval
Commercial Use
New Disease Targeted
What are the opportunities at SMSbiotech for an investor exit
SMSbiotech provides multiple opportunities to investors for exits and cash out on their investments:
- SMSbiotech owns a platform technology aiming for the treatment of multiple indications.
- Licensing, partnerships, and acquisitions provide ample opportunities.
- In the case of an initial public offering (IPO) stocks will be traded freely.
What are the primary risks associated with this investment, and how is the company mitigating them?
Summary
This report reviews SMSbiotech’s key risks and mitigation strategies across regulatory, market, financial, operational, clinical, technological, competitive, strategic, and reputational areas. The company demonstrates proactive planning and strong positioning to support investor confidence and long-term success.
Regulatory Risks
Regulatory approval processes can be complex and lengthy. SMSbiotech mitigates this through expedited Australian trials, early FDA engagement, Compassionate Use approvals, and eligibility for RMAT designation, ensuring faster data generation and regulatory readiness.
Market Risks
Competition and market adoption remain challenges in the COPD and regenerative medicine sectors. SMSbiotech differentiates itself with curative potential, scalable platform applications, strong IP, and entry into high-growth markets with early access opportunities.
Financial Risks
Future funding needs pose risks, given reliance on individual investors to date. The company mitigates this through disciplined spending, early revenue via Compassionate Use, diversified funding strategies, grants, and partnerships.
Operational Risks
Scaling production and supply chain disruptions are potential risks. SMSbiotech addresses these with a patented, scalable manufacturing process, stable distribution methods, quality control systems, and multiple supplier options.
Clinical & Technological Risks
Clinical outcomes and efficacy are inherent risks. Extensive preclinical studies show strong safety and potency, with no adverse reactions, high regeneration rates, and robust delivery methods to maximize therapeutic potential.
Competitive Risks
Competition and IP challenges could impact growth. SMSbiotech counters this with strong patent protection, unique SMS cell advantages, exclusive biomanufacturing capabilities, and a focus on COPD where curative competition is minimal.
Strategic Risks
Misalignment or poor collaborations could slow progress. The company mitigates this through an experienced leadership team, active board oversight, KOL engagement, and carefully structured, incremental partnerships.
Reputation & Perception Risks
Public and investor confidence can be influenced by trial results and media coverage. SMSbiotech actively builds credibility through transparency, frequent updates, conference participation, media engagement, and strong industry relationships.
Disclaimer
The securities referred to in this website may be sold only to accredited investors, which for natural persons, are investors who meet certain minimum annual income or net worth thresholds. These securities are being offered in reliance on an exemption from the registration requirements of the Securities Act and are not required to comply with specific disclosure requirements that apply to registration under the Securities Act. The Securities and Exchange Commission has not passed on the merits of or given its approval to the securities, the terms of the offering, or the accuracy or completeness of any offering materials. The securities are subject to legal restrictions on transfer and resale and investors should not assume that they will be able to resell their securities. Investing in securities involves risk, and investors should be able to bear the loss of their investment.
Safe harbor statement
Matters discussed in this website contain forward-looking statements. When used in this update, the words "anticipate," "believe," "estimate," "may," "intend," "expect" and similar expressions identify such forward-looking statements. Actual results, performance or achievements could differ materially from those contemplated, expressed or implied by the forward-looking statements contained herein. These forward-looking statements are based largely on the expectations of the Company and are subject to a number of risks and uncertainties. These include, but are not limited to, risks and uncertainties associated with our history of losses and our need to raise additional financing, the acceptance of our products and technology in the marketplace, our ability to demonstrate the commercial viability of our products and technology and our need to increase the size of our organization. Further information on the Company's risk factors is contained in the Company's quarterly and annual reports as filed with the Securities and Exchange Commission. The Company undertakes no obligation to revise or update publicly any forward-looking statements for any reason except as may be required under applicable law.

